
Challenges for health systems in Thailand come from various aspects range from impact from demographic change toward aging society, urbanization of Thai population, to the increase in expectation for better public health system due to the advancement in technology together with growth in worldwide trading network. Nevertheless, there is limitation of government budget causing the inadequacy of budget to cover the intensifying public health expenditure. Prompt action is needed in response to such circumstances.
Thailand, therefore, has put medical and public health in the 20-Year National Strategy and set the future goal to promote good health for Thai people and to promote Thailand as a center for international healthcare or the “Medical Hub” by 2036. To help the country attain the position of medical hub, it is necessary to be aware of the current situation of health and medical industry of Thailand in term of health service, medicine, and medical devices. Data from Bank of Thailand shown that value of imported pharmaceutical supplies (medicine, medical supplies, and medical devices) has been continually increased since 2011 – 2015. Signify that, such medical related products are essential to Thai people nowadays as they are tend toward health-conscious lifestyle which benefits the growth in medical and healthcare industry. However, when look at medical industry within the country in response to Medical Hub policy of the Thai government to improve conformation of the product and technology to strengthen country’ competition capabilities, we found that majority of Thai medical products are those low value-added products such as rubber glove, condom, and other consumable medical products which not require advanced technology. On the contrary, Thailand is importing advance technology devices such as X-ray Computerized Tomography, Magnetic Resonance Imaging, etc. To become the international medical hub, Thailand must develop medical products manufacturing sector with advanced technology and innovation in complement with the local demand. Those products, in addition, must also reach both local and international standards which will enable the country to move forward to become the exporter of the medical-related products and reach the country’s goal of becoming the international medical hub.

Business nature of Thailand’s medical devices industry is currently focusing more on trading activities but still lack of technology and inventive creation of its own. The country current medical device manufacturing relies on less complicated manufacturing technology for example in metal products (operation bed, patient bed), dental-related devices (dental unit, electric dental scaler, dental cements), surgical clothing, medical consumable, and middle technology medical devices such as x-ray machine, autoclave, blood pressure monitor, etc.
The more challenge for Thai entrepreneur is to develop product that can transfer technology to the industry. Research and development commercialization of Thailand is quite small in number due to the product research and produced by Thai still lack of credibility. Moreover, in term of product standard, manufacturing facility standard for producing medical device and equipment, after-sales service, warrantee, and market acceptance are major obstacles Thai medical device entrepreneurs still faced.
Prior to promoting of research and development in medical industry such as Medical Robot for Aging, Rehabilitation Assistive Devices, X-ray Imaging/CT, Wearable Sensor Device + AI (as in illustration 3-5), it’s require advance technology integration from various fields including digital x-ray technology, artificial intelligence technology, automation system, sensor system, large-scale data processing system, as well as the network of devices to exchange data via internet (Internet of Things), advance implant material technology, computer-aided design and production technology (CAD/CAM/CNC/3D Printer), and data collection of personal anatomy. To attract foreign investment in manufacturing and R&D of medical industry in Thailand, it is also required development of science and technology infrastructure including animal testing center, performance testing center, clinical testing center, and certify center to upgrade the product standard to meet standard level of FDA and so forth.
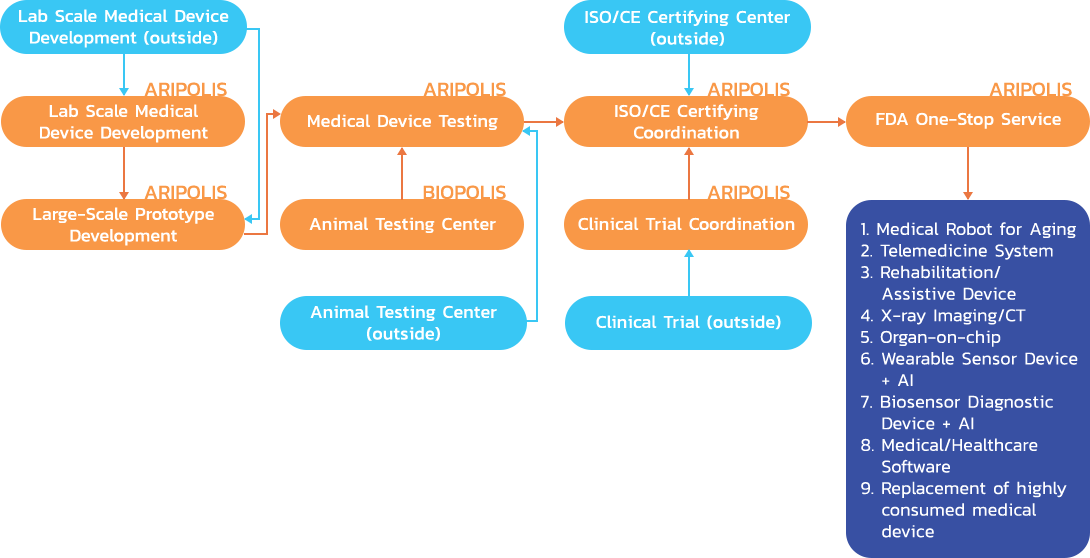
Development Roadmap for Medical Device Industry

Large-Scaled Prototype Development

Animal Testing Center

Medical Device Testing
Home
About EECi
Why EECi?
Vision & Mission
Location
Innovation Ecosystem
Our Growing Partners
Organization Chart
Contact Us
Focused Industries
Modern Agriculture & Advanced Biotechnology
Biofuels & Biochemicals
High Performance Battery & Modern Transports
Automation, Robotics, and Intelligent Electronics
Aviation & Space
Medical Devices
Incentives
Strategic Infrastructure
3-GeV Synchrotron Facility
Biorefinery Pilot Plant
Smart Manufacturing Center
Regulatory Sandbox
National Quality Infrastructure(NQI)
Rental Space
Utilities & Facilities
Main Building
Greenhouse & Plant Factory
Pilot Plant
Leasehold Land
Ecosystem
Talent
Testing
Facilities
Innovation Network
Quality of Life
Publication & Media
News
Factsheet
VDO
Brochure/Poster